People
- Department of Physiology
- About Physiology and Biophysics
-
Education
- Melissa and Pete Shepherd, Jr., PhD New Investigator Prize
- Physiology and Biophysics Educational Programs Home
- Graduate Program
- Undergraduate Research Experience
- Seminar and Lecture Series
- Seminar Series Archives
-
Research
- 2026 Publications
- 2025 Publications
- Physiology and Biophysics Research Home
- Core Facilities
- Programs
- Graduate Program Research
- Publications
- Research Links
- Resources
Hall Research Summary
RESEARCH SUMMARY
Our previous studies suggest that abnormalities of kidney function, manifest by impaired pressure natriuresis, underlie all forms of hypertension studied thus far (click here). In some cases, these disturbances originate intrarenally, but often they occur via activation of neurohumoral mechanisms that impair renal excretory capability. Therefore, a large share of our research has been directed toward understanding the intrarenal and neurohumoral mechanisms that regulate kidney function, and how they are altered in pathophysiologic conditions such as hypertension. Another major emphasis of our laboratory has been on the interaction of hypertension and metabolic risk factors in contributing to target organ injury.
Mechanisms of obesity-induced hypertension and target organ injury.
We have extensively studied obesity-induced hypertension (click here) which may account for 65-75% of the risk for human primary (essential) hypertension. Although the factors that link obesity (especially excess visceral adiposity) with hypertension have not been fully elucidated, kidney dysfunction associated with increased renal sodium reabsorption and compensatory glomerular hyperfiltration play a key role in initiating increased blood pressure and target organ injury. Our previous studies (click here) have elucidated some of the mediators of kidney dysfunction and increased blood pressure including (1) elevated renal sympathetic nerve activity (RSNA); (2) increased antinatriuretic hormones such as angiotensin II and aldosterone; (3) relative deficiency of natriuretic hormones; (4) renal compression by fat in and around the kidneys; and (5) activation of immune mechanisms that produce inflammatory cytokines/chemokines, exacerbating target organ injury and hypertension (Figure 1).
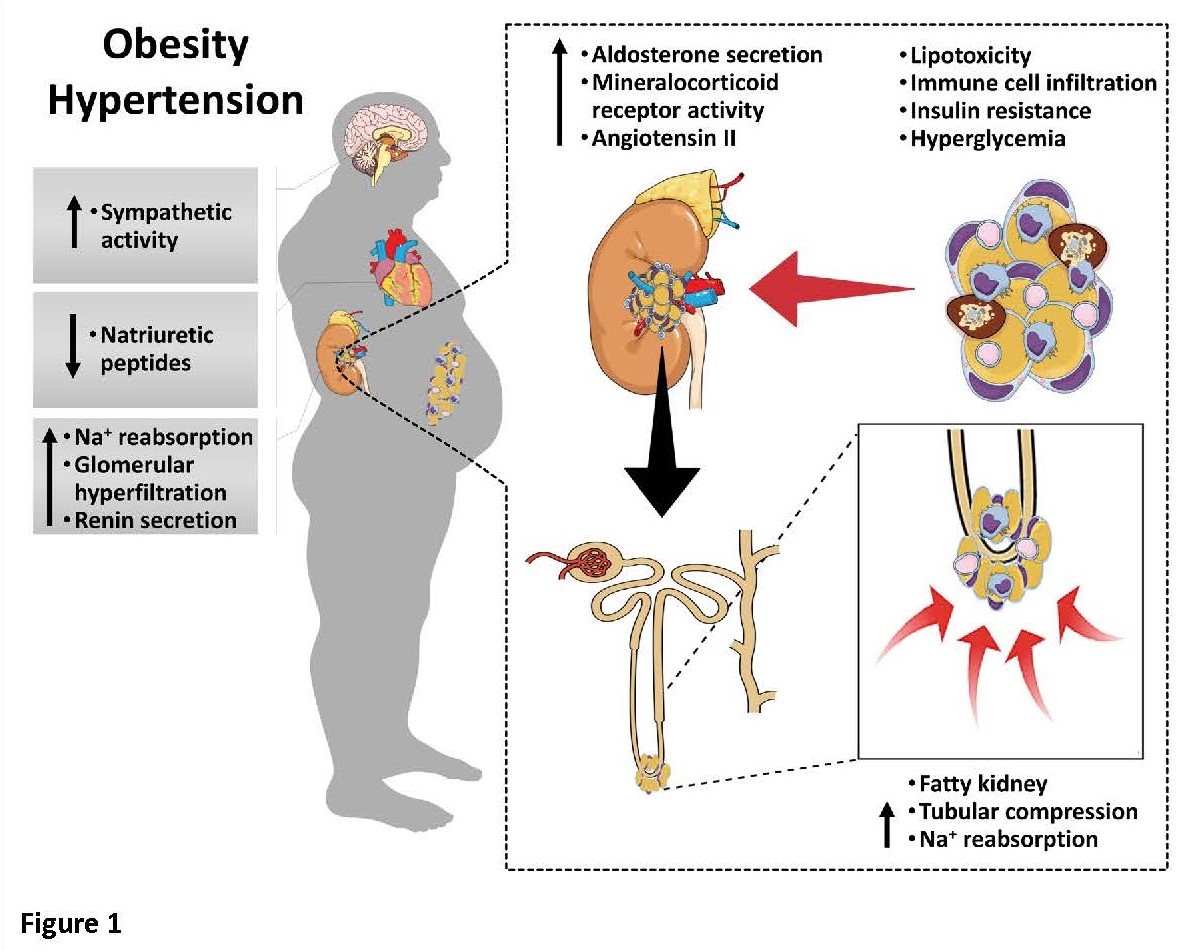
These neurohormonal, renal, and inflammatory mechanisms of obesity-hypertension are interdependent. For example, we have shown that excess adiposity increases adipocyte-derived cytokine leptin which increases RSNA by stimulating the central nervous system proopiomelanocortin (POMC)-melanocortin 4 receptor (MC4R) pathway. Excess visceral, perirenal and renal sinus fat compress the kidneys which, along with increased RSNA, contribute to renin–angiotensin–aldosterone system activation, although obesity may also activate mineralocorticoid receptors independent of aldosterone. Prolonged obesity, hypertension, metabolic abnormalities, and inflammation may cause progressive renal injury, making hypertension more resistant to therapy and often requiring multiple antihypertensive drugs and concurrent treatment of dyslipidemia, insulin resistance, diabetes, and inflammation.
More effective anti-obesity drugs are needed to prevent and treat obesity as well as the cascade of cardiorenal, metabolic, and immune disorders that threaten to overwhelm health care systems as obesity prevalence continues to increase. Therefore, an important aim of our research is to better understand the central nervous system molecular signaling pathways that regulate food intake, energy expenditure and overall energy balance as well as the pathophysiology of obesity-induced hypertension and target organ injury so that more effective therapies can be targeted.
Role of adipocyte-derived leptin and brain melanocortin system in cardiometabolic regulation.
Our previous studies discovered that increases in leptin, a hormone released from adipocytes, cause chronic increases in blood pressure via activation of the sympathetic nervous system. We also used genetic engineering methods to determine how fat cells communicate with the brain to differentially regulate metabolic and cardiovascular functions (Figure 2).
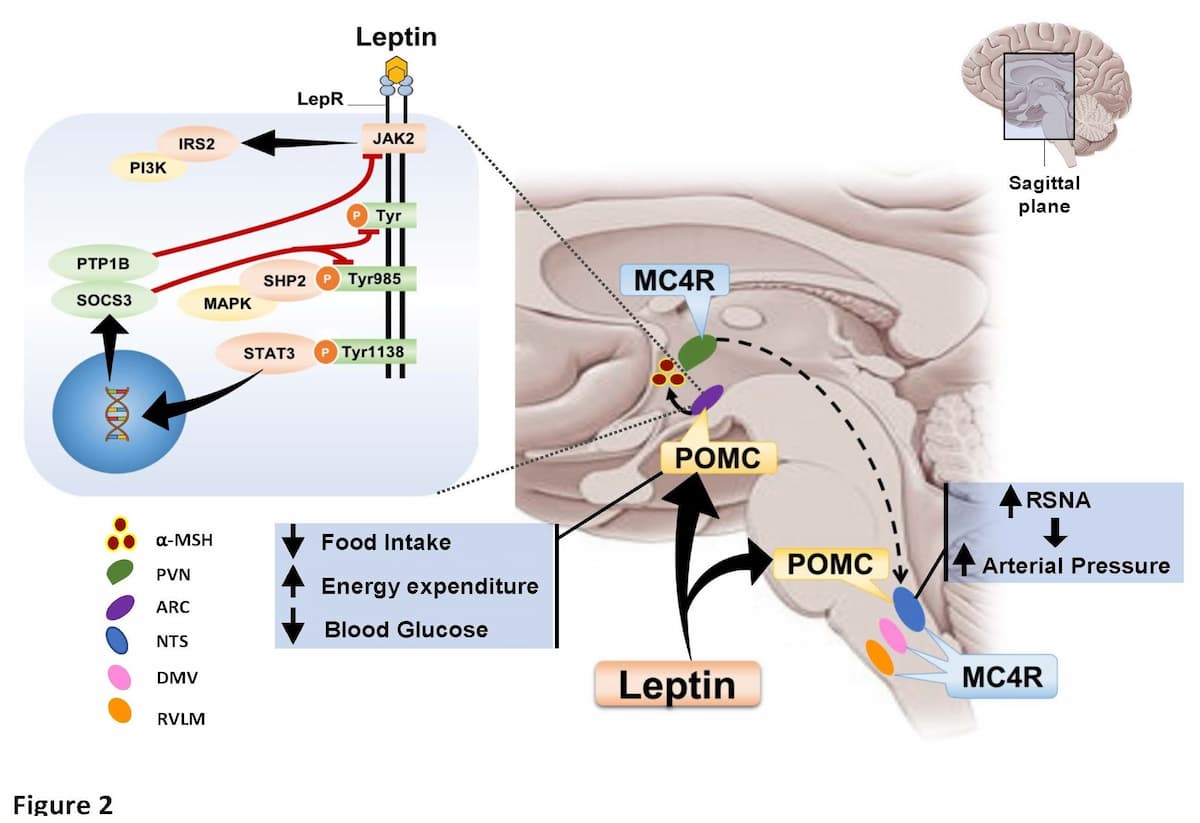
These studies led to the finding that activation of the brain POMC-MC4R pathway not only mediates the effects of leptin on sympathetic activity and blood pressure, but also the powerful antidiabetic effects of leptin as well as the cardioprotective effects of leptin infusion after myocardial infarction. However, the specific neuronal circuits in the brain and the mechanisms by which the brain communicates to peripheral tissues to regulate glucose metabolism and cardiac function are still unknown and the subject of current research in our lab. Our studies suggest that the powerful antidiabetic effects of leptin, capable of completely normalizing blood glucose levels in insulin-deficient animals, may be mediated by non-neural mechanisms, perhaps a neuroendocrine factor released from the brain. Likewise, the mechanisms by which leptin-mediated activation of the brain melanocortin system exerts its cardioprotective actions are unknown and the subject of ongoing studies in our lab (click here).
Our lab uses a wide range of genetic, molecular, integrative physiological, and translational approaches in our research studies. Many of our studies have important implications for understanding basic cardiovascular, renal and endocrine physiology as well as the pathophysiology of obesity, hypertension, kidney disease, and heart failure.


